In July 2018, FDA launched the Biosimilars Motion Plan (BAP), which outlined FDA’s strategy for increasing entry to biosimilars for the American public. The plan centered on 4 key areas:
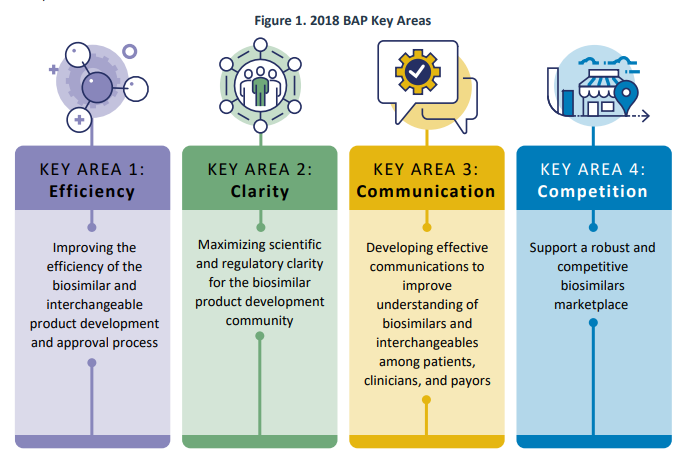
A current FDA report opinions a few of their accomplishments since then. Most of those efforts revolve round steering paperwork, further employees, training merchandise and web sites, public hearings and laws (i.e., proposed/ultimate guidelines). FDA additionally added new knowledge sources together with publishing a modernized model of the Purple Ebook in February 2020. FDA additionally collaborated with different businesses corresponding to FTC, and produced a joint assertion and held a workshop in March 2020, entitled: “Public Workshop: FDA/FTC Workshop on a Competitive Marketplace for Biosimilars.” Another key actions are listed beneath.

The complete report is here.