A pair of coronary heart gadgets linked to a whole lot of accidents and a minimum of 14 deaths has obtained the FDA’s most critical recall, the company announced Monday.
The recall comes years after surgeons say they first seen issues with the HeartMate II and HeartMate 3, manufactured by Thoratec Corp., a subsidiary of Abbott Laboratories. The gadgets usually are not at the moment being faraway from the market. In an emailed response, Abbott mentioned IT had communicated the danger to clients this 12 months.
The delayed motion raises questions for some security advocates about how and when points with authorized medical gadgets ought to be reported. The center gadgets in query have been related to hundreds of studies of sufferers’ accidents and deaths, as described in a KFF Health Information investigation late final 12 months.
“Why doesn’t the general public know?” mentioned Sanket Dhruva, a heart specialist and an knowledgeable in medical machine security and regulation on the College of California-San Francisco. Although some surgeons could have been conscious of points, others, significantly those that don’t implant the machine steadily, could have been at midnight. “And their sufferers are struggling adversarial occasions,” he mentioned.
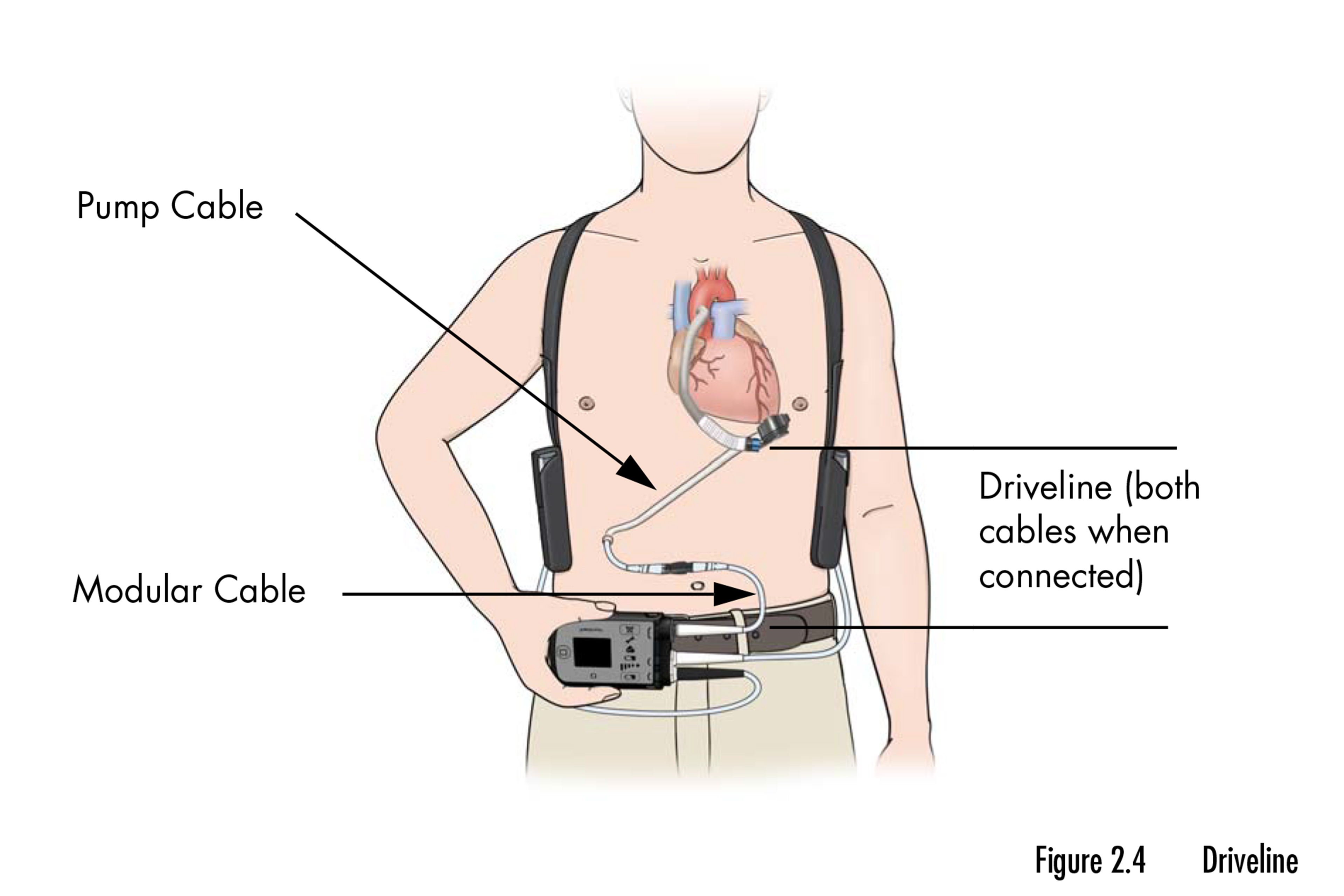
The recall includes a pair of mechanical pumps that assist the center pump blood when IT can’t accomplish that by itself. The gadgets, sufficiently small to slot in the palm of a hand, are implanted in sufferers with end-stage coronary heart failure who’re ready for a transplant or as a everlasting answer when a transplant isn’t an choice. The recall impacts almost 14,000 gadgets.
Amanda Hils, an FDA press officer, mentioned the company is working with Abbott to analyze the reported accidents and deaths and decide if additional motion is required.
“To this point, the variety of deaths reported seems per the adverse events observed in the initial clinical trial,” Hils mentioned in an e-mail.
In response to the FDA’s recall discover, the gadgets could cause buildup of “organic materials” that reduces their capacity to assist the center flow into blood and preserve sufferers alive. The buildup accumulates steadily and may seem two years or extra after a tool is implanted in a affected person’s chest.
Medical doctors have been suggested to be careful for “low-flow alarms” on the gadgets and, in the event that they do diagnose the obstruction, to both monitor the affected person or carry out surgical procedure to implant a stent, launch the blockage, or change the pump. “Charges of outflow obstruction are low,” Abbott spokesperson Justin Paquette mentioned in an e-mail, including that sufferers whose gadgets are functioning usually “don’t have any cause for concern.”
A evaluate of the FDA machine database reveals a minimum of 130 studies associated to HeartMate II or 3 that point out the complication reported by regulators. The earliest such report filed with the FDA dates to a minimum of 2020, in line with a KFF Health Information evaluate of the database.
Monday’s alert is the second Class 1 recall of a HeartMate machine this 12 months.
In January, Abbott issued an pressing “correction letter” to hospitals about a separate issue by which the HeartMate 3 unintentionally begins and stops because of the pump’s communication system, which cardiologists use to evaluate sufferers’ standing. The FDA alerted the public in March.
In February, Abbott issued another urgent letter to hospitals concerning the blockage downside, asking them to tell physicians, full and return an acknowledgment kind, and take note of low-flow alarms on the machine’s monitor which will point out an obstruction. The corporate mentioned within the letter that IT is engaged on “a design answer” to stop the blockages.
A study published in 2022 within the Journal of Thoracic and Cardiovascular Surgical procedure reported the obstruction in about 3% of circumstances, although the incidence charge was larger the longer a affected person had the machine.
The one different Class 1 recall issued for the HeartMate 3 was in Might 2018, when the corporate issued corrective motion notices to hospitals and physicians warning that the graft line that carries blood from the pump to the aorta may twist and cease blood stream.
The FDA recall discover issued Monday contains additional guidance for physicians to diagnose the blockage utilizing an algorithm to detect obstructions and, if wanted, a CT angiogram to confirm the trigger.
At current, the HeartMate 3, which was first authorized by the FDA in 2017, is the one medical choice for a lot of sufferers with end-stage coronary heart failure and who don’t qualify for a transplant. The HeartMate 3 has supplanted the HeartMate II, which obtained FDA approval in 2008.
If the brand new recall results in the machine being faraway from the market, end-stage coronary heart failure sufferers may don’t have any choices, mentioned Francis Pagani, a cardiothoracic surgeon on the College of Michigan who additionally oversees a proprietary database of HeartMate II and HeartMate 3 implants.
If that occurs, “we’re in hassle,” Pagani mentioned. “IT can be devastating to the sufferers to not have this selection. IT’s not an ideal choice — no pump ever is — however that is pretty much as good as IT’s ever been.”
IT’s not identified exactly what number of sufferers have obtained a HeartMate II or HeartMate 3 implant. That Information is proprietary. The FDA recall notices present worldwide distribution of greater than 22,000 HeartMate 3 devices and greater than 2,200 of the HeartMate II.
The blockage complication could have gone unreported to the general public for thus lengthy partly as a result of physicians usually are not required to report adversarial occasions to federal regulators, mentioned Madris Kinard, a former FDA medical machine official and founding father of Device Events, an organization that makes FDA machine knowledge extra user-friendly for hospitals, regulation companies, and buyers.
Solely machine producers, machine importers, and hospitals are required by law to report device-related accidents, deaths, and important malfunctions to the FDA.
“If that is one thing physicians have been conscious of, however they weren’t mandated to report back to the FDA,” Kinard mentioned, “at what level does that communication between these two teams must occur?”
Dhruva, the heart specialist, mentioned he’s in search of transparency from Abbott about what the corporate is doing to handle the issue so he can have extra thorough conversations with sufferers contemplating a HeartMate machine.
“We’re going to count on to have some knowledge saying, ‘Hey we created this repair, and this repair works, and IT doesn’t trigger a brand new downside.’ That’s what I need to know,” he mentioned. “There’s only a ton extra that I really feel at midnight about, to be trustworthy, and I’m certain that sufferers and their households do as nicely.”
[Update: This article was updated at 5:20 p.m. ET on April 16, 2024, with a response from Abbott Laboratories, which IT provided after publication.]